Acetone Polar or Nonpolar Solvent
They can dissolve salts and other ionizable solutes. Polar solutes will dissolve better in polar solvents and nonpolar solutes will dissolve better in nonpolar solvents.
Is Acetone Polar Or Nonpolar Quora
Polar molecules have separated electrical charges on different sides of the molecule.
. Fats have glycerol in addition to three fatty acids. Include use of high solvent power non-polar solvents for lipids. The structure of the fatty acids determines whether or not the fat is.
There are different degrees of polarity and acetone is less polar than water because only part of the acetone molecule has a polar bond. Let this beaker sit beside the water beaker. Why is acetone a better solvent than water.
Given thatBoiling point of the solution 957 CMass of methyl acetate 50Assume that mass of the. DMSO the water is already strongly. Nonpolar solvents include things like toluene gasoline and oils.
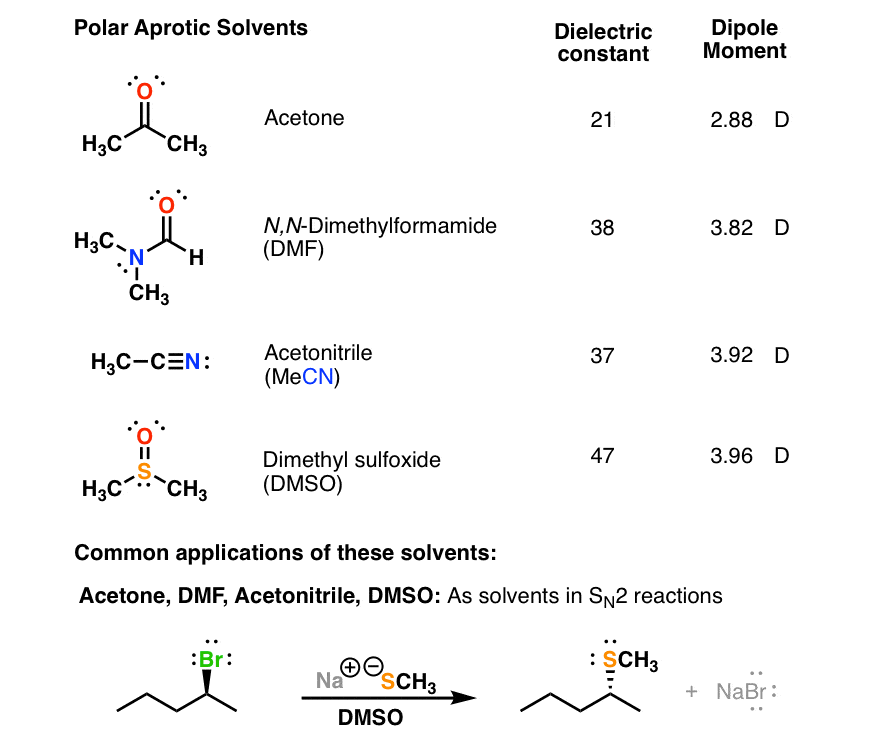
Polar solvents are further classified into polar protic and polar aprotic solvents. This is particularly true for nonpolar solvents. Acetone is a polar solvent used primarily to dissolve fingernail polish.
And use of a low solvent power polar solvents for proteins amino acids and carbohydrates. Water as a solvent dissolves sodium chloride by breaking into ions. Thin-layer chromatography TLC is a chromatography technique used to separate non-volatile mixtures.
It is also produced as a by-product in many large scale production of compounds. That is why chemists use polar aprotic solvents for nucleophilic substitution reactions in the laboratory. Shifts of the solvent residual peak2 and the water peak.
The Rf value varies depending on the solvent used but the general order of the pigments from the highest to the lowest Rf value usually remains the same because the nonpolar compounds move further than the polar compounds. The methanol permeance 2419 liters m 2 hour 1 bar 1 of TAPA-TFP was about four times higher than the existing polyamide-based nanofiltration. Rf values for various pigments using hexane acetone and trichloromethane 311 for the solvent are shown in table 1.
A heterocyclic organic compound. However PE only dissolves at temperatures well above 100 C Polystyrene has a solubility parameter of 91 cal 12 cm 32 and thus ethyl acetate is likely to be a good solvent. They are polar enough to solvate the nucleophile but not so polar as to lock it away in an impenetrable solvent cage.
A powerful psychoactive drug. 327 g mol 1The polyimide membrane with 15-h. Also any potential hydrogen-bond acceptor will tend to shift the water signal down-field.
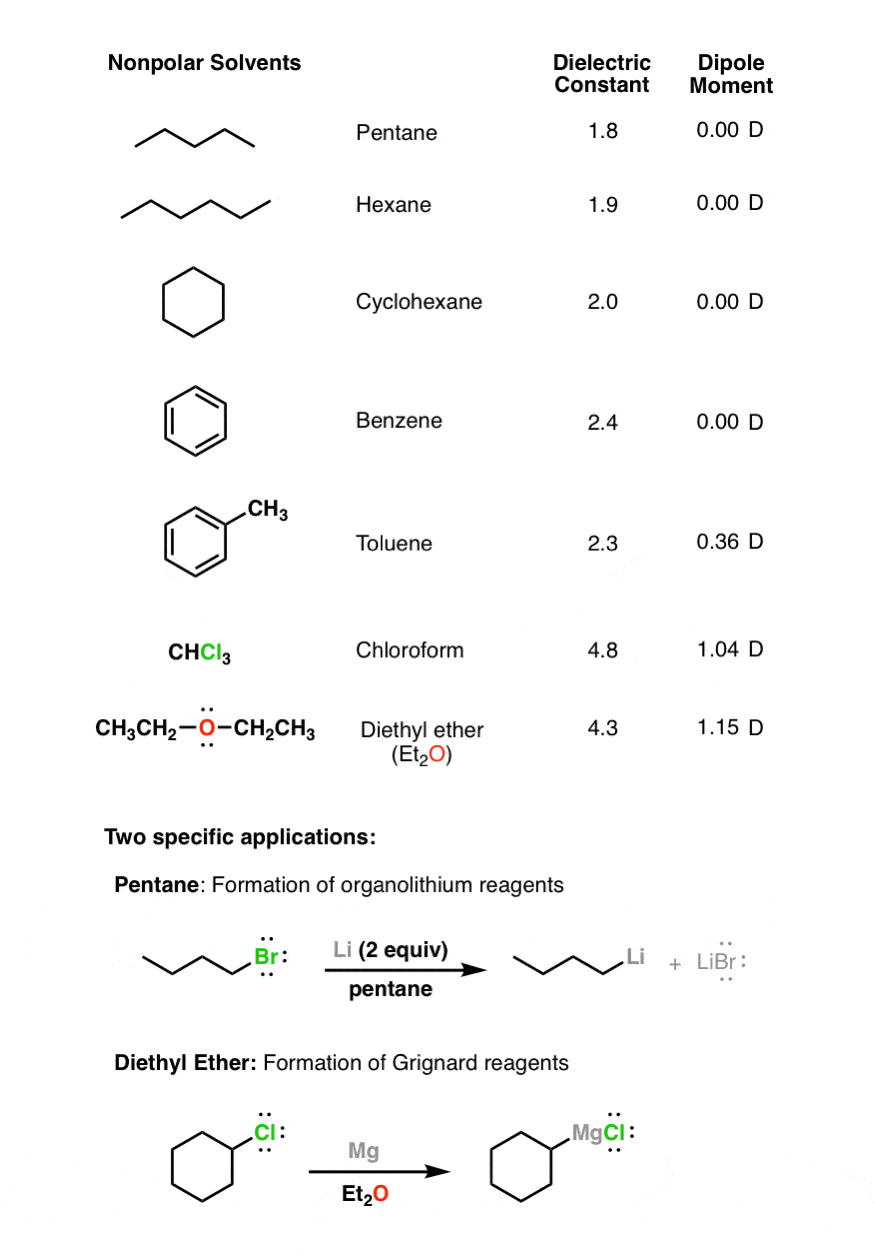
The conductivity of a solution depends on the solvation of its ions. Apolar or nonpolar solvents have low dielectric constants which refers to the electric charge between the molecules being evenly distributed. In addition to acetone three other commonly used polar aprotic solvents are acetonitrile dimethylformamide DMF and.
If acetone could be kept from. It should be noted that the latter is quite temperature-dependent videinfra. Becoming a nonpolar solvent favoring free radical reactions.
SOLVENT MISCIBILITY TABLE. An important polar aprotic solvent that dissolves both polar and nonpolar compounds Dioxane. This does not happen when a nonpolar solvent is added to a polar solvent.
It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. All the ions and proteins in a cell are dissolved in water within the cell. Many nonpolar solvents fall into the category of volatile organic compounds VOCs and are quite dangerous.
Add a nonpolar solvent to a new beaker. Thin-layer chromatography is performed on a sheet of an inert substrate such as glass plastic or aluminium foil which is coated with a thin layer of adsorbent material usually silica gel aluminium oxide alumina or celluloseThis layer of adsorbent is known as the stationary phase. Used in alcoholic beverages in thermometers as a solvent and as a fuel Fehlings reagent.
The key difference between the two is Protic solvents contain at least one hydrogen atom connected directly to a highly electronegative atom such as F N O and thus can make. R - COOR Esters Ethyl acetate R - CO - R Aldehydes Acetone methyl ethyl and ketones ketone R - NH 2 Amines Pyridine triethylamine R - OH Alcohols Methanol ethanol. Secondly since it is miscible acetone is a strong solvent ensuring it has the potential to blend in all amounts with water.
However the latter is polar. The polar solvent dissolves polar solute and the nonpolar solvent Q. Marien and Vankelecom studied the effect of solvent treatment on cross-linked Matrimid polyimide ultrafiltration membranes The immersion of membranes in various solvents was shown to densify the membranes and increase the rejections of low molecular weight dyes such as methyl orange MO MW.
The high-polar inner cavities of TAPA-TFP provide superhighways for polar solvent molecules while the low-polar inner cavities of TAPA-TFB facilitate the transport of nonpolar solvents. These are solvents having a dielectric constant of more than 15. R - COOH Carboxylic acids Ethanoic acid H - OH Water Water Nonpolar Polar Increasing Polarity.
Therefore supercritical water becomes a poor solvent for ionic and highly polar materials at low densities. A single solvent is generally preferred to minimize fractionation and. Polar solvents are often found to have a high dielectric constant although other solvent scales are also used to classify solvent polarity.
More examples of polar molecules Sulfur dioxide SO 2 ammonia NH 3 carbon monoxide CO ethanol C 2 H 5 OH methanol CH 3 OH hydrogen sulfide H 2 S chloromethane CH 3 Cl ozone O3 phosphorus trichloride. Nylon 66 has a solubility parameter of 137 cal 12 cm 32 and ethanol is likely to be the best solvent of those tabulated. Sodium chloride has a NaCl molecule which breaks into Na and Cl- ions when dissolved in water.
Nonpolar molecules while they can fluctuate in charge do not carry a static charge. Classified as an ether Ethanol. Nonpolar solvents cannot solvate ions and ions will.
Add 100 mL of a chosen nonpolar solvent to another beaker. Calculate the percent by mass of methyl acetate in appointing solution around two significant digits A. The most common three types of solvents in organic chemistry are apolar polar aprotic and polar protic.
A solvent from the Latin solvō loosen untie solve is a substance that dissolves a solute resulting in a solutionA solvent is usually a liquid but can also be a solid a gas or a supercritical fluidWater is a solvent for polar molecules and the most common solvent used by living things. Lipids as a class of compounds are insoluble in water but are soluble in other organic solventsExamples of such solvents include acetone and ether. That is solutes typically will dissolve best in solvents that have the most molecular similarities.
ScCO 2 is a promising green solvent because of its negligible toxicity and high solubility window for different kind of solutes. Molecules in general have two classes polar and nonpolar. In contrast in eg.
Dimethyl sulfoxide DMSO is an organosulfur compound with the formula CH 3 2 S OThis colorless liquid is the sulfoxide most widely used commercially. Owing to its ability to dissolve both polar and nonpolar compounds acetone is a strong solvent while other solvents can only dissolve one or the other. Polar solvents can be used to dissolve inorganic or ionic compounds such as salts.
The dielectric constants of water at temperatures of 280 and 300C with pressure of 25 MPa are similar to ethanol and acetone Kritzer and Dinjus 2001. The polarity differentiation is done based on the dielectric constant of the solvents. It has a relatively high boiling point.
Its high diffusivity and variable density in the supercritical region replaced many conventional and toxic solvents 9It provides a green pathway for the many chemical reactions. Waxes steroids phospholipids and fats are the most common types of lipid groups. Additionally solutes will be more soluble if the molecules in the solute are smaller than the ones in the solvent.
In some instances this may be a single solvent or may be a combination of 2 or more solvents in specific ratios. DMSO has the unusual.
Polar Protic Polar Aprotic Nonpolar All About Solvents

Is Acetone Polar Or Nonpolar Techiescientist

Polar Protic Polar Aprotic Nonpolar All About Solvents

Polar Protic Polar Aprotic Nonpolar All About Solvents Organic Chemistry Hydrogen Bond Chemistry
What Makes Acetone A Really Good Solvent What Allows It To Dissolve Both Polar And Non Polar Molecules Quora

Comments
Post a Comment